COMPANY NEWS
Essex Bio-Technology Enters into Global Co-Development Agreement with Mitotech to Fund Phase 3 Clinical Trial in U.S. FDA of a First-In-Class Drug for Dry Eye Disease
2018.07.18
Download
Hong Kong, 16 July 2018
Essex Bio-Technology Limited (“EssexBio” or the “Group”—Stock Code: 1061) is pleased to announce that Essex Bio-Investment, a wholly-owned subsidiary of the Group, entered into the Co-Development Agreement with Mitotech and Russia Mitotech, under which Essex Bio-Investment has agreed to fund a clinical trial development in a U.S. FDA first Phase 3 Clinical Trial of the Product, being a first-in-class ophthalmic solution for the treatment of dry eye disease, in return for a share of certain income received by Mitotech in respect of the Product in accordance with the agreed percentage allocation between Essex Bio-Investment and Mitotech.
Global Co-Development Agreement
Under the agreement, EssexBio is to fund approximately US$17 million (equivalent to approximately HK$133,450,000) in accordance with the agreed funding schedule, for a clinical development in a U.S. FDA first Phase 3 Clinical Trial of the Product, which contains SkQ1 as its sole active pharmaceutical ingredient for the treatment of Dry Eye Disease.
In addition, Essex Bio-Investment has an option to fund a further Development, being the second Phase III Clinical Trial, of the Product to an amount of US$20,000,000 (equivalent to approximately HK$157,000,000).
License Agreement
Based on above Co-Development agreement, Zhuhai Essex Bio-Pharmaceutical Company Limited, an indirectly wholly-owned subsidiary of the Group, will enter into a License Agreement, under which Zhuhai Essex Bio-Pharmaceutical Company Limited will be granted an exclusive, perpetual license of the SkQ1 for the Product to be commercialised in Singapore and the Greater China market (including the People’s Republic of China, Taiwan, Hong Kong and Macau), for treatment of Dry Eye Disease and Uveitis.
The Board of Directors of EssexBio believes that both Co-Development and Licensing Agreement present a good opportunity for the parties to leverage on each other’s Research & Development capability and engineering resources to seek the SkQ1 containing ophthalmic drug for FDA and CFDA approval and for its commercialisation globally.
Forming this strategic partnership with Mitotech is a pragmatic approach enabling EssexBio to strengthen its market position in ophthalmology within China and initiate its expansion into the global marketplace.
About Mitotech S.A.
Mitotech S.A. is a clinical-stage Luxembourg-based biotechnology company developing novel drugs for the treatment of predominantly age-related disorders. The core technology behind Mitotech products is based on a novel class of small molecules – mitochondria targeting cardiolipin peroxidation inhibitors. Company’s lead compound SkQ1 is being developed in several drug formulations covering a variety of therapeutic areas with major focus on ophthalmology and neurodegenerative diseases. Mitotech successfully completed Phase II clinical study for Dry Eye Disease in the U.S. with other indications also approaching clinical stage of development.
About Dry Eye Disease (DED)
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a multi-factorial chronic and potentially debilitating disease affecting the lacrimal functional unit including the ocular surface. DED may lead to altered composition of tear film (tear film instability) which fails to support ocular epithelial health, resulting in potential damage to ocular surface (cornea), promoting ocular surface inflammation. As a consequence, DED can hinder the ability to effectively carry out daily activities, with a negative impact on quality of life. Epidemiology data (Table 1) suggests a high prevalence rate of DED resulting from an aging population and changes in lifestyle (of all ages) due to increased dependency on on-screen technology, a concern that DED is becoming a prevalent disease throughout the world.
China in particular has a high prevalence with varying differences geographically within China, it is estimated that 75 million people suffer from DED in China3.
With stepwise therapeutic approaches for DED patients limited to artificial tears and anti-inflammation, coupled with a lack of consensus in standard measurement for signs and symptoms, and effective treatment for DED, there is an increasing concern and need for better therapeutics to assist in ophthalmologists’ practice. This is especially evident in China, with treatment of DED reaching US$145.6 million in 2018, despite the high prevalence, and despite global DED market reaching US$5.04 billion in 2016 and estimated to reach US$7.78 billion by the end of 20253,4.
Development of optimized and feasible treatment with novel agents and compounds will address such unmet medical needs and address DED’s underlying pathophysiology.
Table 1
Essex Bio-Technology Limited (“EssexBio” or the “Group”—Stock Code: 1061) is pleased to announce that Essex Bio-Investment, a wholly-owned subsidiary of the Group, entered into the Co-Development Agreement with Mitotech and Russia Mitotech, under which Essex Bio-Investment has agreed to fund a clinical trial development in a U.S. FDA first Phase 3 Clinical Trial of the Product, being a first-in-class ophthalmic solution for the treatment of dry eye disease, in return for a share of certain income received by Mitotech in respect of the Product in accordance with the agreed percentage allocation between Essex Bio-Investment and Mitotech.
Global Co-Development Agreement
Under the agreement, EssexBio is to fund approximately US$17 million (equivalent to approximately HK$133,450,000) in accordance with the agreed funding schedule, for a clinical development in a U.S. FDA first Phase 3 Clinical Trial of the Product, which contains SkQ1 as its sole active pharmaceutical ingredient for the treatment of Dry Eye Disease.
In addition, Essex Bio-Investment has an option to fund a further Development, being the second Phase III Clinical Trial, of the Product to an amount of US$20,000,000 (equivalent to approximately HK$157,000,000).
License Agreement
Based on above Co-Development agreement, Zhuhai Essex Bio-Pharmaceutical Company Limited, an indirectly wholly-owned subsidiary of the Group, will enter into a License Agreement, under which Zhuhai Essex Bio-Pharmaceutical Company Limited will be granted an exclusive, perpetual license of the SkQ1 for the Product to be commercialised in Singapore and the Greater China market (including the People’s Republic of China, Taiwan, Hong Kong and Macau), for treatment of Dry Eye Disease and Uveitis.
The Board of Directors of EssexBio believes that both Co-Development and Licensing Agreement present a good opportunity for the parties to leverage on each other’s Research & Development capability and engineering resources to seek the SkQ1 containing ophthalmic drug for FDA and CFDA approval and for its commercialisation globally.
Forming this strategic partnership with Mitotech is a pragmatic approach enabling EssexBio to strengthen its market position in ophthalmology within China and initiate its expansion into the global marketplace.
About Mitotech S.A.
Mitotech S.A. is a clinical-stage Luxembourg-based biotechnology company developing novel drugs for the treatment of predominantly age-related disorders. The core technology behind Mitotech products is based on a novel class of small molecules – mitochondria targeting cardiolipin peroxidation inhibitors. Company’s lead compound SkQ1 is being developed in several drug formulations covering a variety of therapeutic areas with major focus on ophthalmology and neurodegenerative diseases. Mitotech successfully completed Phase II clinical study for Dry Eye Disease in the U.S. with other indications also approaching clinical stage of development.
About Dry Eye Disease (DED)
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a multi-factorial chronic and potentially debilitating disease affecting the lacrimal functional unit including the ocular surface. DED may lead to altered composition of tear film (tear film instability) which fails to support ocular epithelial health, resulting in potential damage to ocular surface (cornea), promoting ocular surface inflammation. As a consequence, DED can hinder the ability to effectively carry out daily activities, with a negative impact on quality of life. Epidemiology data (Table 1) suggests a high prevalence rate of DED resulting from an aging population and changes in lifestyle (of all ages) due to increased dependency on on-screen technology, a concern that DED is becoming a prevalent disease throughout the world.
China in particular has a high prevalence with varying differences geographically within China, it is estimated that 75 million people suffer from DED in China3.
With stepwise therapeutic approaches for DED patients limited to artificial tears and anti-inflammation, coupled with a lack of consensus in standard measurement for signs and symptoms, and effective treatment for DED, there is an increasing concern and need for better therapeutics to assist in ophthalmologists’ practice. This is especially evident in China, with treatment of DED reaching US$145.6 million in 2018, despite the high prevalence, and despite global DED market reaching US$5.04 billion in 2016 and estimated to reach US$7.78 billion by the end of 20253,4.
Development of optimized and feasible treatment with novel agents and compounds will address such unmet medical needs and address DED’s underlying pathophysiology.
Table 1
| Country | Prevalence rate1,2,3,4,5 |
| U.S. | 7.0% |
| Europe | 14.0% to 33.0% |
| Singapore | 12.3% |
| China | 21.0% to 30.0% |
| Worldwide | 5.0% to 34.0% |
About SkQ1
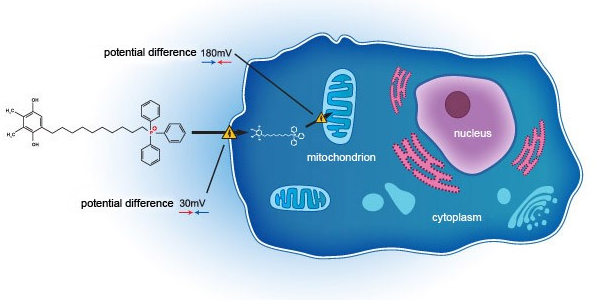
SkQ1 addresses DED through a novel mechanism of action, acting on the mitochondria on a cellular level. Unlike current standard of care, which acts primarily as anti-inflammatory agents, SkQ1 has been shown to not only relieve inflammation but also improve tissue degeneration and tear quality deficit, by targeting oxidative stress within the eye. Oxidative stress – a phenomenon that is widely evident in patients suffering from DED, regardless of pathophysiology, due to build up of Reactive Oxygen Species (ROS) within the mitochondria. Further, tear hyperosmolarity plays a central role in ocular surface damage related to DED, whilst oxidative stress is an important contributing factor to hyperosmolarity.
Developed as a novel first in class novel molecule through completed clinical phase II study in the United States (NCT02121301), SkQ1 has been shown to target and neutralise mitochondrial ROS to restore cellular function, downregulating TNF-α and IL-6 with pronounced upregulation of IL-10 in tears. SkQ1 demonstrated superiority over placebo in several endpoints, with protective benefit for DED by reducing oxidative stress within mitochondria and improving both signs and symptoms for DED patients.
Mitotech has received regulatory approval for Visomitin (ophthalmic eye drop containing SkQ1) in Russia for the treatment of DED, with approximately 1 million units sold since it was commercially launched. “Our clinical studies showed that Visomitin protected cornea from damage, improve quality of tear and made patients feel better at the same time,” said Natalia Perekhvatova, Chief Executive Officer of Mitotech. “We are getting very positive feedback, from both physicians and patients.”
“We are excited to have established a strategic partnership with Mitotech to co-develop SkQ1 on the global stage, through a Phase III clinical study in the United States,” said Malcolm Ngiam, President of Essex Bio-Investment, “Mitotech recognises Essex Bio-Technology’s strong presence in China and the region, with strengths in research, clinical development, sales and marketing, by entrusting us to develop the product, and we look forward to SkQ1 cementing our position as a leader in Dry Eye Disease and Ophthalmology.”
1 Clin Exp Optom 2015; 98:45-52
2 Clin J Ophthalmol 2013; 49:73-75
3 Data Source: Market Scope, 2018 China Ophthalmology Market Report
4 Data Source: Transparent Market Research, 2018 Dry Eye Disease Market
5 Ophthalmic Epidem 2006; 13:263-274






























































 粤公网安备 44049102496184号
粤公网安备 44049102496184号