COMPANY NEWS
Essex Bio-Technology Announces First Patient First Visit in U.S. FDA Phase 3 Clinical Trial of SkQ1 Occurred on 27 October 2018
2018.10.29
Download
Hong Kong, 29 October 2018
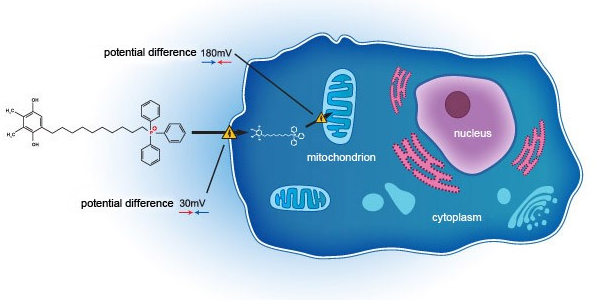
Essex Bio-Technology Limited (“EssexBio” or the “Group”—Stock Code: 1061) is pleased to announce that the co-development partnership between Essex Bio-Investment, a wholly owned subsidiary of the Group, and Mitotech S.A. (“Mitotech”), a Luxembourg-based clinical-stage biotechnology company, in relation to a clinical development in a U.S. FDA Phase 3 clinical trial of SkQ1 compound in patients with moderate to severe Dry Eye Disease (“DED”) has been successfully initiated and the first visit by the first patient occurred on 27 October 2018 (U.S. time). SkQ1 is Mitotech’s lead compound belongs to the class of cardiolipin peroxidation inhibitors that it is developing for treatment of age-related disorders. This is a unique and novel class of molecules targeting mitochondria and designed to protect cells from oxidative stress caused by mtROS. Mitotech’s most clinically advanced formulation is Visomitin – ophthalmic solution of the lead compound developed for several age-related eye disorders, including DED.
"We are truly delighted to be part of this exciting journey and the celebrations of VISTA-1 achieving an important milestone of First Patient First Visit”, said Malcolm Ngiam, President of Essex Bio-Investment. “We look forward to SkQ1 demonstrating its novel mechanism of action and efficacy as a first-in-class ophthalmic solution for DED patients."
VISTA-1 is a multi-centre, randomized, double-blind, placebo-controlled clinical study involving three treatment arms: two concentrations of SkQ1 and placebo administered BID. Approximately 450 patients will be enrolled in the study across multiple centres in the U.S. and will receive treatment over a 2-month period. VISTA-1 aims at confirming positive effect of SkQ1 on both signs and symptoms of DED observed in previous clinical studies conducted by Mitotech. The study is also designed to confirm excellent safety, tolerability and comfort profile of the drug observed during previous studies and during patient experience in Russia, where the drug has been marketed since 2012.
"We are very excited to open this new chapter of clinical development for our lead drug candidate”, said Natalia Perekhvatova, Chief Executive Officer of Mitotech S.A.. “Development of a drug platform with innovative mechanism of action is certainly a challenging process, and I am happy that our team reached this important milestone. We are looking forward to receiving results of the study in the second quarter of 2019 and if the study is successful, Visomitin may become a significant first in class treatment option for patients suffering from DED.”
"We have put a lot of effort into designing this study”, said Lawrence Friedhoff, Chief Clinical Officer of Mitotech S.A.. “It is carefully engineered to highlight particular aspects of the innovative mechanism of action of SkQ1 in addition to confirming its effect on signs and symptoms of DED. We are also hoping to reliably demonstrate fast onset of action for our drug, which is an important attribute for a modern DED treatment.”
More information about VISTA-1 clinical trial will be available at www.clinicaltrials.gov soon.
About Dry Eye Disease
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a multi-factorial chronic and potentially debilitating disease affecting the lacrimal functional unit including the ocular surface. DED may lead to altered composition of tear film (tear film instability) which fails to support ocular epithelial health, resulting in potential damage to ocular surface (cornea), promoting ocular surface inflammation. As a consequence, DED can hinder the ability to effectively carry out daily activities, with a negative impact on quality of life. Aging population and changes in lifestyle (of all ages) due to increased dependency on on-screen technology trigger a concern that DED is becoming a prevalent disease throughout the world.
Currently EssexBio has Beifushu series and Single-dose Sodium Hyaluronate Eye Drops, both products are therapeutically approved for the treatment of DED with different mechanism of action. The expected launch of Visomitin in U.S. and China will significantly strengthen the Group’s pipeline for the treatment of DED, enhancing the Group’s leading position for ophthalmology business in China and achieving expansion to overseas market in terms of ocular drugs.
About Mitotech S.A.
Mitotech S.A. is a Luxembourg-based biotechnology company developing novel drugs for treatment of predominantly age-related disorders. The core technology behind Mitotech products is based on a novel class of small molecules – mitochondria targeting cardiolipin peroxidation inhibitors. Company’s lead compound SkQ1 is being developed in several drug formulations covering a variety of therapeutic areas with major focus on ophthalmology and neurodegenerative diseases. Mitotech successfully completed Phase 2 clinical study for Dry Eye indication in the U.S. with other indications also approaching clinical stage of development.
Essex Bio-Technology Limited (“EssexBio” or the “Group”—Stock Code: 1061) is pleased to announce that the co-development partnership between Essex Bio-Investment, a wholly owned subsidiary of the Group, and Mitotech S.A. (“Mitotech”), a Luxembourg-based clinical-stage biotechnology company, in relation to a clinical development in a U.S. FDA Phase 3 clinical trial of SkQ1 compound in patients with moderate to severe Dry Eye Disease (“DED”) has been successfully initiated and the first visit by the first patient occurred on 27 October 2018 (U.S. time). SkQ1 is Mitotech’s lead compound belongs to the class of cardiolipin peroxidation inhibitors that it is developing for treatment of age-related disorders. This is a unique and novel class of molecules targeting mitochondria and designed to protect cells from oxidative stress caused by mtROS. Mitotech’s most clinically advanced formulation is Visomitin – ophthalmic solution of the lead compound developed for several age-related eye disorders, including DED.
"We are truly delighted to be part of this exciting journey and the celebrations of VISTA-1 achieving an important milestone of First Patient First Visit”, said Malcolm Ngiam, President of Essex Bio-Investment. “We look forward to SkQ1 demonstrating its novel mechanism of action and efficacy as a first-in-class ophthalmic solution for DED patients."
VISTA-1 is a multi-centre, randomized, double-blind, placebo-controlled clinical study involving three treatment arms: two concentrations of SkQ1 and placebo administered BID. Approximately 450 patients will be enrolled in the study across multiple centres in the U.S. and will receive treatment over a 2-month period. VISTA-1 aims at confirming positive effect of SkQ1 on both signs and symptoms of DED observed in previous clinical studies conducted by Mitotech. The study is also designed to confirm excellent safety, tolerability and comfort profile of the drug observed during previous studies and during patient experience in Russia, where the drug has been marketed since 2012.
"We are very excited to open this new chapter of clinical development for our lead drug candidate”, said Natalia Perekhvatova, Chief Executive Officer of Mitotech S.A.. “Development of a drug platform with innovative mechanism of action is certainly a challenging process, and I am happy that our team reached this important milestone. We are looking forward to receiving results of the study in the second quarter of 2019 and if the study is successful, Visomitin may become a significant first in class treatment option for patients suffering from DED.”
"We have put a lot of effort into designing this study”, said Lawrence Friedhoff, Chief Clinical Officer of Mitotech S.A.. “It is carefully engineered to highlight particular aspects of the innovative mechanism of action of SkQ1 in addition to confirming its effect on signs and symptoms of DED. We are also hoping to reliably demonstrate fast onset of action for our drug, which is an important attribute for a modern DED treatment.”
More information about VISTA-1 clinical trial will be available at www.clinicaltrials.gov soon.
About Dry Eye Disease
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a multi-factorial chronic and potentially debilitating disease affecting the lacrimal functional unit including the ocular surface. DED may lead to altered composition of tear film (tear film instability) which fails to support ocular epithelial health, resulting in potential damage to ocular surface (cornea), promoting ocular surface inflammation. As a consequence, DED can hinder the ability to effectively carry out daily activities, with a negative impact on quality of life. Aging population and changes in lifestyle (of all ages) due to increased dependency on on-screen technology trigger a concern that DED is becoming a prevalent disease throughout the world.
Currently EssexBio has Beifushu series and Single-dose Sodium Hyaluronate Eye Drops, both products are therapeutically approved for the treatment of DED with different mechanism of action. The expected launch of Visomitin in U.S. and China will significantly strengthen the Group’s pipeline for the treatment of DED, enhancing the Group’s leading position for ophthalmology business in China and achieving expansion to overseas market in terms of ocular drugs.
About Mitotech S.A.
Mitotech S.A. is a Luxembourg-based biotechnology company developing novel drugs for treatment of predominantly age-related disorders. The core technology behind Mitotech products is based on a novel class of small molecules – mitochondria targeting cardiolipin peroxidation inhibitors. Company’s lead compound SkQ1 is being developed in several drug formulations covering a variety of therapeutic areas with major focus on ophthalmology and neurodegenerative diseases. Mitotech successfully completed Phase 2 clinical study for Dry Eye indication in the U.S. with other indications also approaching clinical stage of development.






























































 粤公网安备 44049102496184号
粤公网安备 44049102496184号