COMPANY NEWS
EssexBio awarded “2021 China Pharmaceutical Industry Top 100 Series List” and “2022 China Pharmaceutical Brands List”
2022.07.15
Download
Hong Kong, 15 July 2022
About the "2021 Top 100 Chinese Chemical Pharmaceutical Companies Ranking”
About Beifushu®
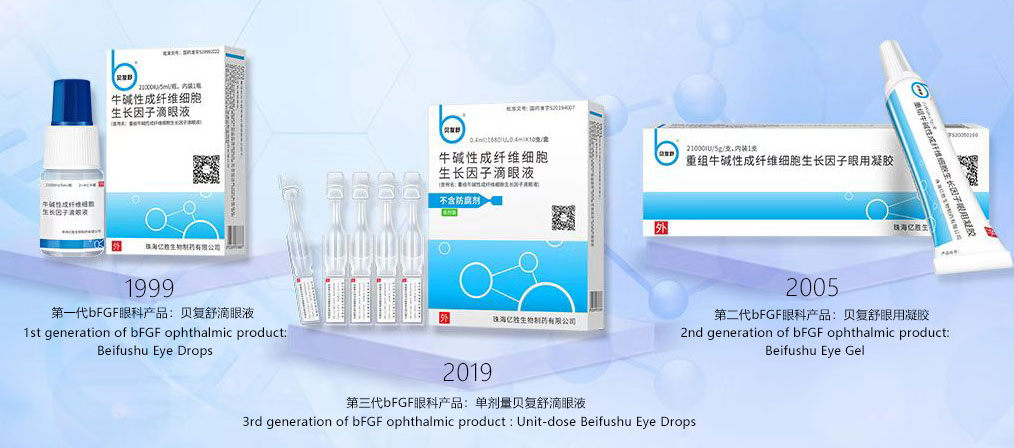
Beifushu® is a national first-in-class biologics based on b-bFGF, and is also the world’s first marketed growth factor ophthalmic preparation. It is mainly used to treat various corneal injuries and diseases, and is widely used in post-surgery recovery and to treat dry eye disease. It is currently the first-line medication for the treatment of ocular surface repair. Beifushu® has been available in the market for more than 20 years. Pursuing continuous innovation based on the ophthalmic clinical [trials] and the development of drug technology, Beifushu® has developed the third-generation product, which has substantially satisfied the clinical treatment needs of different ophthalmological patients. Beifushu® has earned a good reputation and trust by clinicians and patients alike, ranking among the top ophthalmic drugs in terms of sales volume.
About Essex (1061.HK)
Essex Bio-Technology Ltd (“Essex” or the “Group”, Stock Code: 1061.HK) is pleased to announce that Zhuhai Essex Bio-Pharmaceutical Company Limited(“Zhuhai Essex”), an indirect wholly-owned subsidiary, has once again been awarded “2021 China Pharmaceutical Industry Top 100 Series List” assessed by Menet, climbing 20 places over the previous year. The Group’s flagship product Beifushu® was also awarded “2022 Chinese Pharmaceutical Brands List”, in the ophthalmic category, for the fourth consecutive year.




We are honoured to receive recognition for our continued contribution to the industry and the field of Ophthalmology. Essex will continue to strive for excellence by embracing innovation to develop first-in-class and best-in-class products, providing solutions for Tomorrow’s healthcare problems, Today.
~ End ~
About the "2021 Top 100 Chinese Chemical Pharmaceutical Companies Ranking”
The "2021 Top 100 Chinese Chemical Pharmaceutical Companies Ranking" is a subset of the "2021 China Pharmaceutical Industry Top 100 Series List". It mainly assesses two criteria: "innovation driver" and "professional marketing". While the former is the integrated benchmark score of R&D investment in 2021, and the latter is graded based on the quantitative analysis of the data of three major terminals of Menet and six other terminals in the market. Hengrui Pharmaceuticals, Sino Biopharmaceutical and Shanghai Pharma were the top three in the list.
About the “2022 China Pharmaceutical Brands List”The “2022 China Pharmaceutical Brands List” has rigourously screened the shortlisted brands based on the sales amount and growth rate of products in the past three years (2019-2021) as shown by the data from Menet's three terminals, namely the “public hospital terminal”, “grassroots medical terminal” and the “retail pharmacy terminal”. Moreover, a WeChat voting session was arranged to allow a wider group of professional readers to select the finalists from the shortlisted brands. This year, a total of 128 pharmaceutical brands were included on the list.
About Beifushu®

Beifushu® is a national first-in-class biologics based on b-bFGF, and is also the world’s first marketed growth factor ophthalmic preparation. It is mainly used to treat various corneal injuries and diseases, and is widely used in post-surgery recovery and to treat dry eye disease. It is currently the first-line medication for the treatment of ocular surface repair. Beifushu® has been available in the market for more than 20 years. Pursuing continuous innovation based on the ophthalmic clinical [trials] and the development of drug technology, Beifushu® has developed the third-generation product, which has substantially satisfied the clinical treatment needs of different ophthalmological patients. Beifushu® has earned a good reputation and trust by clinicians and patients alike, ranking among the top ophthalmic drugs in terms of sales volume.
Essex Bio-Technology Limited is a bio-pharmaceutical company that develops, manufactures and commercialises genetically engineered therapeutic b-bFGF (FGF-2), having six commercialised biologics marketed in China since 1998. Additionally, it has a portfolio of commercialised products of preservative-free unit-dose eye drops and Shilishun(適麗順®)(Iodized Lecithin Capsules) etc.. The products of the Company are principally prescribed for the treatment of wounds healing and diseases in Ophthalmology and Dermatology, which are marketed and sold through approximately 10,500 hospitals and managed directly by its 43 regional sales offices in China. Leveraging on its in-house R&D platform in growth factor and antibody, the Company maintains a pipeline of projects in various clinical stages, covering a wide range of fields and indications.






























































 粤公网安备 44049102496184号
粤公网安备 44049102496184号