COMPANY NEWS
Essex Bio-Technology and Mitotech Announce Topline Results from VISTA-1, Phase 3 Clinical Trial in U.S. FDA of a First-In-Class Drug for Dry Eye Disea
2019.07.19
Download
Hong Kong, 19 July 2019
Essex Bio-Technology Limited (“EssexBio” or the “Group” or the “Company”, Stock code: 1061) and Mitotech S.A. (“Mitotech”) today announced that topline results from a Phase 2b/3 study, VISTA-1, a clinical development in U.S.FDA for SkQ1 ophthalmic solution (NCT03764735) , a first-in-class drug designed for the treatment of dry eye syndrome.
Topline data from VISTA-1 showed encouraging time to efficacy for a spectrum of clinically relevant signs and symptoms, in spite of not meeting co-primary endpoints (Central Corneal Fluorescein Staining and Grittiness). Relative to the Vehicle, SkQ1 demonstrated statistically significant reduction of Ocular Discomfort (p<0.05) as early as after 4 weeks of treatment with symptoms in 4-Symptom Questionnaire also demonstrating statistically significant reduction (p<0.05), in the Intent To Treat (ITT) population. Conjunctival fluorescein staining also demonstrated statistically significant reduction vs. Vehicle (p<0.05), also in ITT population. The study highlighted excellent safety profile of the drug with tolerability being statistically similar to that of artificial tear.
“The manifestation of positive VISTA-1 results not only provided valuable guidance for the regulatory path, but also demonstrated significant product differentiation for the DED market.” Said Quinn Xue, Chief Scientific Officer of Essex Bio-Technology Limited.
“This data set speaks for itself”, said Natalia Perekhvatova, Chief Executive Officer of Mitotech S.A. “VISTA-1 results revealed early onset of action of SkQ1 for a spectrum of clinically relevant symptoms and signs such as Ocular Discomfort and Fluorescein Staining. Combined with drug tolerability comparable to that of an artificial tear, SkQ1 is well-poised to become an important potential treatment option for DED patients worldwide.”
“After having seen clear positive signal in topline data, we begin preparation for a follow-on programme, VISTA-2”, said Ms. Perekhvatova “Developing a drug with innovative mechanism of action in DED has its challenges and this is why we are excited to see broad action of SkQ1 in VISTA- 1 results with statistically significant impact on a variety of metrics for both signs and symptoms. Taking current options for DED treatments into account, excellent drug tolerability is also a great asset for both companies.”
The Company is encouraged by the robust data on efficacy and safety shown in VISTA-1 and will continue further data evaluation. In view of the positive trial data obtained during the first phase 3 clinical trial, EssexBio and Mitotech are discussing on taking the U.S.FDA Phase 3 Clinical Trial to the next step i.e. Second Phase 3 Clinical Trial.

About VISTA-1
VISTA-1 is a multi-center, double-blind, randomized, placebo-controlled study comprising 5 visits over the course of approximately 9 weeks. Qualified subjects (approximately 450) are randomized 1:1:1 to receive either high dose SkQ1 ophthalmic solution(1.55ug/mL), low dose SkQ1 ophthalmic solution(0.155ug/mL), or placebo (vehicle of SkQ1 ophthalmic solution).
The primary endpoints of the study are 8-week change from baseline in central corneal fluorescein staining and 8-week change from baseline in grittiness symptom. Secondary outcome measures included various symptoms, corneal fluorescein staining, lissamine green staining, tear film break-up time, Schirmer's test and conjunctival redness measured both environmentally and during controlled adverse environment challenge (Day 57 only).
About Dry Eye Disease (DED)
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a multi-factorial chronic and potentially debilitating disease affecting the lacrimal functional unit including the ocular surface. DED may lead to altered composition of tear film (tear film instability) which fails to support ocular epithelial health, resulting in potential damage to ocular surface (cornea), promoting ocular surface inflammation. Consequently, DED can hinder the ability to effectively carry out daily activities, with a negative impact on quality of life. With stepwise therapeutic approaches for DED patients limited to artificial tears and anti-inflammation, coupled with a lack of consensus in standard measurement for signs and symptoms, and effective treatment for DED, DED remains a highly underserved clinical arena. According to Transparent Market Research, the global DED market reached US$5.04 billion in 2016 and was estimated to reach US$7.78 billion by the end of 2025.
About SkQ1
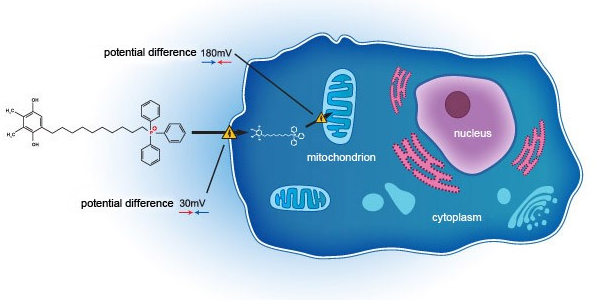
SkQ1 addresses DED through a novel mechanism of action, acting on the mitochondria on a cellular level. Unlike current standard of care, which acts primarily as anti-inflammatory agents, SkQ1 has been shown to not only relieve inflammation but also improve tissue degeneration and tear quality deficit by targeting oxidative stress within the eye. In a previous Phase 2 study in the United States (NCT02121301) SkQ1 showed evidence of efficacy in reducing both the signs and symptoms in dry eye subjects.
About Mitotech S.A.
Mitotech S.A. is a clinical-stage Luxembourg-based biotechnology company developing novel drugs for the treatment of predominantly age-related disorders. The core technology behind Mitotech products is based on a novel class of small molecules – mitochondria targeting cardiolipin peroxidation inhibitors. Company’s lead compound SkQ1 is being developed in several drug formulations covering a variety of therapeutic areas with major focus on ophthalmology and neurodegenerative diseases. Mitotech has received regulatory approval for Visomitin (ophthalmic eye drop containing SkQ1) in Russia for the treatment of Dry Eye Syndrome.
Essex Bio-Technology Limited (“EssexBio” or the “Group” or the “Company”, Stock code: 1061) and Mitotech S.A. (“Mitotech”) today announced that topline results from a Phase 2b/3 study, VISTA-1, a clinical development in U.S.FDA for SkQ1 ophthalmic solution (NCT03764735) , a first-in-class drug designed for the treatment of dry eye syndrome.
Topline data from VISTA-1 showed encouraging time to efficacy for a spectrum of clinically relevant signs and symptoms, in spite of not meeting co-primary endpoints (Central Corneal Fluorescein Staining and Grittiness). Relative to the Vehicle, SkQ1 demonstrated statistically significant reduction of Ocular Discomfort (p<0.05) as early as after 4 weeks of treatment with symptoms in 4-Symptom Questionnaire also demonstrating statistically significant reduction (p<0.05), in the Intent To Treat (ITT) population. Conjunctival fluorescein staining also demonstrated statistically significant reduction vs. Vehicle (p<0.05), also in ITT population. The study highlighted excellent safety profile of the drug with tolerability being statistically similar to that of artificial tear.
“The manifestation of positive VISTA-1 results not only provided valuable guidance for the regulatory path, but also demonstrated significant product differentiation for the DED market.” Said Quinn Xue, Chief Scientific Officer of Essex Bio-Technology Limited.
“This data set speaks for itself”, said Natalia Perekhvatova, Chief Executive Officer of Mitotech S.A. “VISTA-1 results revealed early onset of action of SkQ1 for a spectrum of clinically relevant symptoms and signs such as Ocular Discomfort and Fluorescein Staining. Combined with drug tolerability comparable to that of an artificial tear, SkQ1 is well-poised to become an important potential treatment option for DED patients worldwide.”
“After having seen clear positive signal in topline data, we begin preparation for a follow-on programme, VISTA-2”, said Ms. Perekhvatova “Developing a drug with innovative mechanism of action in DED has its challenges and this is why we are excited to see broad action of SkQ1 in VISTA- 1 results with statistically significant impact on a variety of metrics for both signs and symptoms. Taking current options for DED treatments into account, excellent drug tolerability is also a great asset for both companies.”
The Company is encouraged by the robust data on efficacy and safety shown in VISTA-1 and will continue further data evaluation. In view of the positive trial data obtained during the first phase 3 clinical trial, EssexBio and Mitotech are discussing on taking the U.S.FDA Phase 3 Clinical Trial to the next step i.e. Second Phase 3 Clinical Trial.

About VISTA-1
VISTA-1 is a multi-center, double-blind, randomized, placebo-controlled study comprising 5 visits over the course of approximately 9 weeks. Qualified subjects (approximately 450) are randomized 1:1:1 to receive either high dose SkQ1 ophthalmic solution(1.55ug/mL), low dose SkQ1 ophthalmic solution(0.155ug/mL), or placebo (vehicle of SkQ1 ophthalmic solution).
The primary endpoints of the study are 8-week change from baseline in central corneal fluorescein staining and 8-week change from baseline in grittiness symptom. Secondary outcome measures included various symptoms, corneal fluorescein staining, lissamine green staining, tear film break-up time, Schirmer's test and conjunctival redness measured both environmentally and during controlled adverse environment challenge (Day 57 only).
About Dry Eye Disease (DED)
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a multi-factorial chronic and potentially debilitating disease affecting the lacrimal functional unit including the ocular surface. DED may lead to altered composition of tear film (tear film instability) which fails to support ocular epithelial health, resulting in potential damage to ocular surface (cornea), promoting ocular surface inflammation. Consequently, DED can hinder the ability to effectively carry out daily activities, with a negative impact on quality of life. With stepwise therapeutic approaches for DED patients limited to artificial tears and anti-inflammation, coupled with a lack of consensus in standard measurement for signs and symptoms, and effective treatment for DED, DED remains a highly underserved clinical arena. According to Transparent Market Research, the global DED market reached US$5.04 billion in 2016 and was estimated to reach US$7.78 billion by the end of 2025.
About SkQ1
SkQ1 addresses DED through a novel mechanism of action, acting on the mitochondria on a cellular level. Unlike current standard of care, which acts primarily as anti-inflammatory agents, SkQ1 has been shown to not only relieve inflammation but also improve tissue degeneration and tear quality deficit by targeting oxidative stress within the eye. In a previous Phase 2 study in the United States (NCT02121301) SkQ1 showed evidence of efficacy in reducing both the signs and symptoms in dry eye subjects.
About Mitotech S.A.
Mitotech S.A. is a clinical-stage Luxembourg-based biotechnology company developing novel drugs for the treatment of predominantly age-related disorders. The core technology behind Mitotech products is based on a novel class of small molecules – mitochondria targeting cardiolipin peroxidation inhibitors. Company’s lead compound SkQ1 is being developed in several drug formulations covering a variety of therapeutic areas with major focus on ophthalmology and neurodegenerative diseases. Mitotech has received regulatory approval for Visomitin (ophthalmic eye drop containing SkQ1) in Russia for the treatment of Dry Eye Syndrome.





























































 粤公网安备 44049102496184号
粤公网安备 44049102496184号