COMPANY NEWS
Essex Bio-Technology (1061.HK) 2022 Annual Results at a glance
2023.03.08

Related news
 Essex Bio-Technology establishes strategic collaboration with Biosparc Signs MOU with SIPIPO and Biosparc on International Innovation Accelerator
Essex Bio-Technology establishes strategic collaboration with Biosparc Signs MOU with SIPIPO and Biosparc on International Innovation Accelerator
2025-11-21
 Resilient Interim Results: Essex Bio-Technology Drives Growth and Innovation, Revenue up 5.8% to HK$876.5 Million, Profit up 3.8% to HK$ 163.4 million, 16.7% Dividend Surge, Continued Innovation Momentum in Regulatory Milestones
Resilient Interim Results: Essex Bio-Technology Drives Growth and Innovation, Revenue up 5.8% to HK$876.5 Million, Profit up 3.8% to HK$ 163.4 million, 16.7% Dividend Surge, Continued Innovation Momentum in Regulatory Milestones
2025-08-26
 NMPA Accepted Essex’s Biologics License Application for EB12-20145P (HLX04-O) for the Treatment of Wet Age-Related Macular Degeneration.
NMPA Accepted Essex’s Biologics License Application for EB12-20145P (HLX04-O) for the Treatment of Wet Age-Related Macular Degeneration.
2025-08-13
 Multi-Dose Diquafosol Sodium Eye Drops Obtained Approval from NMPA for Commercialisation in China
Multi-Dose Diquafosol Sodium Eye Drops Obtained Approval from NMPA for Commercialisation in China
2025-07-24
 Study Primary Endpoint Met in a Phase 3 Clinical Study of Bevacizumab EB12-20145P (HLX04-O) for the Treatment of Ophthalmic Diseases
Study Primary Endpoint Met in a Phase 3 Clinical Study of Bevacizumab EB12-20145P (HLX04-O) for the Treatment of Ophthalmic Diseases
2025-04-02
 Essex Bio-Technology Reports Stellar 2024 Annual Results:Net Profit soars 11.6% to HK$ 307.2 Million, Dividend increases 33.3% Focuses on Strengthening R&D Capabilities and Expanding Market Access
Essex Bio-Technology Reports Stellar 2024 Annual Results:Net Profit soars 11.6% to HK$ 307.2 Million, Dividend increases 33.3% Focuses on Strengthening R&D Capabilities and Expanding Market Access
2025-03-26
 Preservative-free Unit-Dose Sodium Hyaluronate Eye Drops (0.3%) Obtained Approval from NMPA for Commercialisation in China
Preservative-free Unit-Dose Sodium Hyaluronate Eye Drops (0.3%) Obtained Approval from NMPA for Commercialisation in China
2024-09-13
 Essex Bio-Technology Reports 1st H 2024 Net Profit of HK$157.4 Million Poised for Future Success Underpinned by Proven Product Base and Promising Pipeline of Products
Essex Bio-Technology Reports 1st H 2024 Net Profit of HK$157.4 Million Poised for Future Success Underpinned by Proven Product Base and Promising Pipeline of Products
2024-08-26
 Preservative-free Unit-Dose Diquafosol Sodium Eye Drops Obtained Approval from NMPA for Commercialisation in China
Preservative-free Unit-Dose Diquafosol Sodium Eye Drops Obtained Approval from NMPA for Commercialisation in China
2024-08-14
 EssexBio has climbed 13 places over the previous year in “China Pharmaceutical Industry Top 100 Series List” & Beifushu® awarded “China Pharmaceutical Brands List” for the sixth consecutive year
EssexBio has climbed 13 places over the previous year in “China Pharmaceutical Industry Top 100 Series List” & Beifushu® awarded “China Pharmaceutical Brands List” for the sixth consecutive year
2024-06-27
 Essex Bio-Technology Posts Sound 2023 Annual Financial Results: Revenue Up 29.5%, Profit Up 22.1%
Essex Bio-Technology Posts Sound 2023 Annual Financial Results: Revenue Up 29.5%, Profit Up 22.1%
2024-03-18
 Essex Bio-Technology Posts Sound 2023 Interim Financial Results Revenue Up 37.1%, Profit Up 22%
Essex Bio-Technology Posts Sound 2023 Interim Financial Results Revenue Up 37.1%, Profit Up 22%
2023-08-16
 The phase 1/2 clinical trial of Bevacizumab for treatment of Ophthalmic Diseases completed
The phase 1/2 clinical trial of Bevacizumab for treatment of Ophthalmic Diseases completed
2023-07-26
 EssexBio awarded “2022 China Pharmaceutical Industry Top 100 Series List” and “2023 China Pharmaceutical Brands List”
EssexBio awarded “2022 China Pharmaceutical Industry Top 100 Series List” and “2023 China Pharmaceutical Brands List”
2023-06-30
 Essex and Osteopore Entered into Exclusive Distribution Agreement for Oral Maxillofacial products in Singapore
Essex and Osteopore Entered into Exclusive Distribution Agreement for Oral Maxillofacial products in Singapore
2023-04-14
 Essex and Gunze Shenzhen Entered into Exclusive Agency Agreement for PELNAC® Absorbable Dressing in Mainland China
Essex and Gunze Shenzhen Entered into Exclusive Agency Agreement for PELNAC® Absorbable Dressing in Mainland China
2023-04-11
 Essex Bio-Technology Announces 2022 Annual Financial Results, Resilience & Relevance, Growth Ready
Essex Bio-Technology Announces 2022 Annual Financial Results, Resilience & Relevance, Growth Ready
2023-03-08
 Essex and Henlius signed amendment agreement for Global Co-Development and Exclusive License Agreement for treatment of age-related macular degeneration
Essex and Henlius signed amendment agreement for Global Co-Development and Exclusive License Agreement for treatment of age-related macular degeneration
2023-02-22
 First Patient in the US Dosed in a Global Multicentre Phase 3 Clinical Study of Bevacizumab for treatment of Ophthalmic Diseases
First Patient in the US Dosed in a Global Multicentre Phase 3 Clinical Study of Bevacizumab for treatment of Ophthalmic Diseases
2023-02-10
 Essex Biotechnology Secures Exclusive Global Rights and Interests of SkQ1 in the field Ophthalmology from Mitotech
Essex Biotechnology Secures Exclusive Global Rights and Interests of SkQ1 in the field Ophthalmology from Mitotech
2022-10-13
 EssexBio awarded “2021 China Pharmaceutical Industry Top 100 Series List” and “2022 China Pharmaceutical Brands List”
EssexBio awarded “2021 China Pharmaceutical Industry Top 100 Series List” and “2022 China Pharmaceutical Brands List”
2022-07-15
 Essex Bio-Technology attends the 2022 GRC-FGF Conference, with two cutting-edge scientific research accepted by GRC
Essex Bio-Technology attends the 2022 GRC-FGF Conference, with two cutting-edge scientific research accepted by GRC
2022-05-07
 First Patient in Australia Dosed in a Global Multicentre Phase 3 Clinical Study of Bevacizumab for treatment of Ophthalmic Diseases
First Patient in Australia Dosed in a Global Multicentre Phase 3 Clinical Study of Bevacizumab for treatment of Ophthalmic Diseases
2022-04-19
 First Patient Dosed in a Global Multi-Centre Phase 3 Clinical Study of Bevacizumab EB12-20145P (HLX04-O) for treatment of Ophthalmic Diseases in the EU
First Patient Dosed in a Global Multi-Centre Phase 3 Clinical Study of Bevacizumab EB12-20145P (HLX04-O) for treatment of Ophthalmic Diseases in the EU
2022-04-08
 Essex Bio-Technology Announces 2021 Financial Results Achieves an increase of 58% in Profit-After-Tax to HK$346.0 million and Turnover Growth of 67.4% to HK$1,637.7 million
Essex Bio-Technology Announces 2021 Financial Results Achieves an increase of 58% in Profit-After-Tax to HK$346.0 million and Turnover Growth of 67.4% to HK$1,637.7 million
2022-03-22
 Essex Bio-Technology Wins "Best Investment Value Award for Listed Companies" under China Securities Golden Bauhinia Awards Gains Wide Recognition from Capital Market for Comprehensive Strength and Investment Value
Essex Bio-Technology Wins "Best Investment Value Award for Listed Companies" under China Securities Golden Bauhinia Awards Gains Wide Recognition from Capital Market for Comprehensive Strength and Investment Value
2021-12-20
 First Patient First Visit Completed in the PRC in a Phase 3 Clinical Trial of Bevacizumab EB12-20145P (HLX04-O)
First Patient First Visit Completed in the PRC in a Phase 3 Clinical Trial of Bevacizumab EB12-20145P (HLX04-O)
2021-11-10
 Essex Bio-Technology to Present at the 5th China Bio-Pharm Partnering Forum (Bio-Pharm 2021)
Essex Bio-Technology to Present at the 5th China Bio-Pharm Partnering Forum (Bio-Pharm 2021)
2021-10-27
 First Patient First Visit Completed in the PRC in Clinical Trial of Bevacizumab EB12-20145P (HLX04-O)
First Patient First Visit Completed in the PRC in Clinical Trial of Bevacizumab EB12-20145P (HLX04-O)
2021-07-19
 Application for Clinical Trial of Bevacizumab Has Been Approved for the Treatment of wAMD in Latvia
Application for Clinical Trial of Bevacizumab Has Been Approved for the Treatment of wAMD in Latvia
2021-04-20
 Preservative-free Unit-dose Moxifloxacin Hydrochloride Eye Drops Obtained Approval from NMPA for Commercialisation in China
Preservative-free Unit-dose Moxifloxacin Hydrochloride Eye Drops Obtained Approval from NMPA for Commercialisation in China
2021-04-07
 Essex-Biotechnology Announces Bevacizumab Has Received IND Approval from US FDA for the Treatment of wAMD
Essex-Biotechnology Announces Bevacizumab Has Received IND Approval from US FDA for the Treatment of wAMD
2021-03-19
 Essex Bio-Technology Announces 2020 Financial Results Sales of HK$654m & PAT of HK$170m recorded in 2nd Half
Essex Bio-Technology Announces 2020 Financial Results Sales of HK$654m & PAT of HK$170m recorded in 2nd Half
2021-03-12
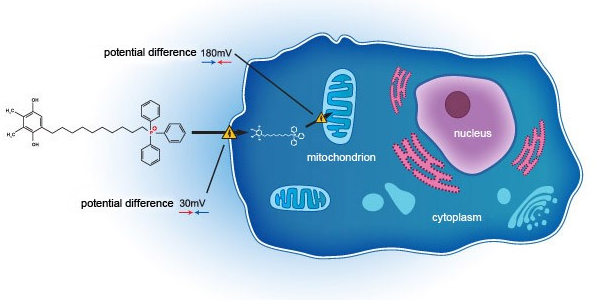 Essex Bio-Technology and Mitotech announce positive outcome of VISTA-2 Phase 3 clinical study in Dry Eye Disease
Essex Bio-Technology and Mitotech announce positive outcome of VISTA-2 Phase 3 clinical study in Dry Eye Disease
2021-02-24
 Essex-Biotechnology Announces Bevacizumab for wAMD Has Received Clinical Trial Approval in Australia
Essex-Biotechnology Announces Bevacizumab for wAMD Has Received Clinical Trial Approval in Australia
2021-01-29
 Essex and Henlius Entered into a Global Co-Development and Exclusive License Agreement to Jointly Develop Bevacizumab for Treatment of Ophthalmic Diseases
Essex and Henlius Entered into a Global Co-Development and Exclusive License Agreement to Jointly Develop Bevacizumab for Treatment of Ophthalmic Diseases
2020-10-15
 Essex Bio-Technology and Mitotech Complete Enrollment in VISTA-2 – a Pivotal Phase 3 Clinical Study of SkQ1 for Dry Eye Disease
Essex Bio-Technology and Mitotech Complete Enrollment in VISTA-2 – a Pivotal Phase 3 Clinical Study of SkQ1 for Dry Eye Disease
2020-08-25
 Preservative-free Single-dose rb-bFGF Eye Drops (Single-Dose Beifushu Eye Drops) Obtained Approval from NMPA for Commercialisation in China
Preservative-free Single-dose rb-bFGF Eye Drops (Single-Dose Beifushu Eye Drops) Obtained Approval from NMPA for Commercialisation in China
2019-12-27
 Essex Bio-Technology and Mitotech Announce First Patient First Visit in U.S. FDA Second Phase 3 Clinical Trial of SkQ1
Essex Bio-Technology and Mitotech Announce First Patient First Visit in U.S. FDA Second Phase 3 Clinical Trial of SkQ1
2019-12-12
 Admission of Product to the PRC National Drug List for Reimbursement, Beifuxin Newly Listed and the Other Three Products Remain on the List
Admission of Product to the PRC National Drug List for Reimbursement, Beifuxin Newly Listed and the Other Three Products Remain on the List
2019-08-21
 Essex Bio-Technology and Antikor Biopharma Forge Strategic Alliance in FDC for Cancer Treatment
Essex Bio-Technology and Antikor Biopharma Forge Strategic Alliance in FDC for Cancer Treatment
2019-08-02
 Essex Bio-Technology and Mitotech Announce Topline Results from VISTA-1, Phase 3 Clinical Trial in U.S. FDA of a First-In-Class Drug for Dry Eye Disea
Essex Bio-Technology and Mitotech Announce Topline Results from VISTA-1, Phase 3 Clinical Trial in U.S. FDA of a First-In-Class Drug for Dry Eye Disea
2019-07-19
 Essex Bio-Technology Announces Upcoming Joint Presentation with Mitotech and Ora at ARVO 2019 Annual Meeting
Essex Bio-Technology Announces Upcoming Joint Presentation with Mitotech and Ora at ARVO 2019 Annual Meeting
2019-04-29
 FY18 Turnover & Net Profit grew 30.8% and 38.1% to HK$1,176.5 million & HK$ 231.1 million, respectively
FY18 Turnover & Net Profit grew 30.8% and 38.1% to HK$1,176.5 million & HK$ 231.1 million, respectively
2019-03-12
 The cellular growth factor project of Essex Bio-Technology Limited was honoured with the second prize of National Scientific and Technology Progress Award
The cellular growth factor project of Essex Bio-Technology Limited was honoured with the second prize of National Scientific and Technology Progress Award
2019-01-10
 Essex Bio-technology Entered into Convertible Notes Purchase Agreement and License Agreement with DB Therapeutics
Essex Bio-technology Entered into Convertible Notes Purchase Agreement and License Agreement with DB Therapeutics
2018-10-30
2018-10-29
2018-08-22
2018-07-24
2018-07-18
2018-04-12
2018-04-10
























 粤公网安备 44049102496184号
粤公网安备 44049102496184号