COMPANY NEWS
Essex Bio-Technology Announces 2022 Interim Financial Results
2022.08.23
Download
Hong Kong, 23 Aug 2022
Essex Bio-Technology Ltd (“Essex” or the “Group”, Stock Code: 1061.HK) today announced the interim results for the six months ended 30 June 2022.
Financial Performance
During the period under review, the sporadic emergence of COVID-19 in different parts of China continued to add pressure on the Group’s business operation and financial performance. The measures imposed by some provincial and municipal governments in China to curb the spread of COVID-19 had a significant impact on the outpatient operations of hospitals and reduced non-emergency patient visits to hospitals and clinics.
For the six months ended 30 June 2022, the Group achieved a consolidated turnover of approximately HK$655.8 million, and the profit is approximately HK$139.2 million.
The Board has declared an interim dividend of HK$0.04 (For the six months ended 30 June 2021: HK$0.04) per ordinary share for the six months ended 30 June 2022.
Turnover of Ophthalmology and Surgical Segments
The Group’s turnover is primarily made up from the segments of Ophthalmology and Surgical (wound care and healing). The core products that are of current growth driver under each segment are:
Essex Bio-Technology Ltd (“Essex” or the “Group”, Stock Code: 1061.HK) today announced the interim results for the six months ended 30 June 2022.
Financial Performance
During the period under review, the sporadic emergence of COVID-19 in different parts of China continued to add pressure on the Group’s business operation and financial performance. The measures imposed by some provincial and municipal governments in China to curb the spread of COVID-19 had a significant impact on the outpatient operations of hospitals and reduced non-emergency patient visits to hospitals and clinics.
For the six months ended 30 June 2022, the Group achieved a consolidated turnover of approximately HK$655.8 million, and the profit is approximately HK$139.2 million.
The Board has declared an interim dividend of HK$0.04 (For the six months ended 30 June 2021: HK$0.04) per ordinary share for the six months ended 30 June 2022.
Turnover of Ophthalmology and Surgical Segments
The Group’s turnover is primarily made up from the segments of Ophthalmology and Surgical (wound care and healing). The core products that are of current growth driver under each segment are:
- Ophthalmology – Beifushu series (Beifushu eye drops, Beifushu eye gel and Beifushu unit-dose eye drops), Tobramycin Eye Drops, Levofloxacin Eye Drops, Sodium Hyaluronate Eye Drops and 适丽顺® (Iodized Lecithin Capsules); and
- Surgical (wound care and healing) – Beifuji series (Beifuji spray, Beifuji lyophilised powder and Beifuxin gel), Carisolv® dental caries removal gel, 伢典醫生 (Dr. YaDian) mouth wash and伊血安顆粒 ( Yi Xue An Granules).
The sectoral turnover of Ophthalmology and Surgical is approximately HK$269.4 million or 41.1% and HK$386.4 million or 58.9% of the Group’s turnover, respectively.
Significant Business Development Activities
Essex is committed to pragmatically investing in new products and technologies to strengthen the Group’s product and R&D pipeline as near to mid-term growth driver in ophthalmology and long-term plan for new therapeutics in oncology. Major investments during the review period in ophthalmic products that are under advanced stage of clinical development are outlined as follows:
Investments in Ophthalmology
Major Progress in a Global Phase 3 Clinical Study for EB12-20145P (HLX04-O)
In 2020, the Group started co-development on a recombinant anti-VEGF humanised monoclonal antibody (EB12-20145P) for the treatment of wet-AMD, with Shanghai Henlius Biotech, Inc. During the period, EB12-20145P has since been approved to commence phase 3 clinical trials in various territories including (Australia, China, European Union, Singapore and the United States of America.), with the first patients dosed in Australia, China and the European Union.
Successful Completion of the适丽顺® (Iodized Lecithin Capsules) Acquisition
During the period under review, The Group has completed the acquisition of intellectual property rights relating to technologies and process of product research and development, production and right of Marketing Authorisation Holder of适丽顺® (Iodized Lecithin Capsules). 适丽顺® (Iodized Lecithin Capsules) is being regarded as one of the Group’s core products since then.
Honours and Awards Obtained In 1H 2022
In August 2022, Essex was included in “Forbes Asia’s 200 Best Under A Billion 2022” - for the Group’s outstanding long-term sustainable development across a variety of metrics. Only 200 companies were selected out of 20,000 publicly traded companies (with annual sales of between $10 million and $1 billion) in the Asia-Pacific region. The 200 companies were carefully picked based on Forbes’ List Methodology of quantitative criteria and qualitative screens.
In addition, Zhuhai Essex Bio-Pharmaceutical Company Limited (珠海億勝生物製藥有限公司), a wholly-owned subsidiary of the Group, has been recognised as one of the 2021 top 10 pharmaceutical and health manufacturing companies in Zhuhai (2021年度珠海市醫藥健康製造業十強企業). It has also been recognised as one of the 2021 top 100 chemical pharmaceutical companies in the PRC (2021年度TOP100中國化藥企業).In the same period, the Group’s Beifushu has been awarded as one of the Chinese reputable medicine brands in four consecutive years, which is a testament of the recognition by the industry for the efficacy and quality of our flagship biologic drugs.
Market Development
Stable Market Access Capability
Over the years, the Group has been relentlessly investing in establishing and strengthening its market access capability. As of 30 June 2022, the Group maintains a network of 43 regional sales offices in the PRC and a total number of about 1,255 sales and marketing representatives.
Robust Medical Providers Network for the Therapeutic Products
During the period under review, the Group’s therapeutic products are being prescribed in more than 10,710 hospitals and medical providers, coupled with approximately 2,120 pharmaceutical stores, which are widely located in the major cities, provinces and county cities in the PRC.
Strategic investments for competitiveness and a wider customers base
For achieving a sustainable traction on growth for currently marketed products as well as for near-term to mid-term new products being commercialised, the Group initiated investments to improve its competitiveness and widen its customers base under the following plans:
- Investing in clinical observation programmes for affirming additional clinical indications of its commercialised products;
- Reaching out to market in lower-tier cities;
- Cultivating pharmaceutical stores, where possible, as complementary sales channel; and
- Building on-line platform for medical consultation and e-prescription for patients with chronic diseases under its healthtech initiative.
Research and Development
The Blow-Fill-Seal (“BFS”) platform established by the Group, has formed part of the Group’s core competency to develop and produce a series of preservative-free unit-dose drugs. As of 30 June 2022, the Group has 5 commercialised preservative-free unit-dose eye drops in the product pipeline. A handful of preservative-free unit-dose ophthalmic drugs are under development with targeted commercialisation within the next 2 to 5 years.
Currently, there are 15 R&D programmes in the pre-clinical to clinical stage, out of which 3 ophthalmology programmes are in clinical stage. The 3 ophthalmology programmes listed below are targeted as mid-term growth driver:
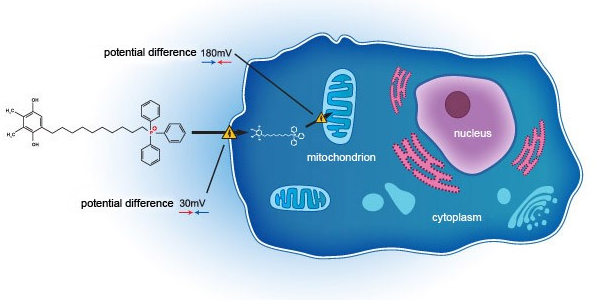
- EB11-18136P: SkQ1 eye drops, second phase 3 clinical trial (US FDA) (VISTA-2) topline data released on 24 February 2021
- EB11-15120P: Azithromycin eye drops, ongoing review by external key opinion leaders (National Medical Products Administration (“NMPA”) in the PRC)
- EB12-20145P: Bevacizumab intravitreal injection for wet-AMD, phase 3 clinical trial (US FDA, European Medicines Agency, Therapeutic Goods Administration and NMPA in the PRC)
As of the date of this announcement, the Group has obtained a total of 64 patent certificates or authorisation letters: 47 invention patents (發明專利), 12 utility model patents (實用新型專利) and 5 design patents (外觀專利).
Forward-Looking Statement
The sporadic emergence of COVID-19 in the PRC if persists will adversely influence our performance in the second half of 2022, despite the strong fundamentals and demands of our products in the market.
~ End ~
About Essex Bio-Technology Limited (Stock Code: 1061.HK)
Essex Bio-Technology Limited is a bio-pharmaceutical company that develops, manufactures and commercialises genetically engineered therapeutic b-bFGF (FGF-2), having six commercialised biologics marketed in China since 1998. Additionally, it has a portfolio of commercialised products of preservative-free unit-dose eye drops and 适丽顺®(Iodized Lecithin Capsules) etc.. The products of the Company are principally prescribed for the treatment of wounds healing and diseases in Ophthalmology and Dermatology, which are marketed and sold through approximately 10,710 hospitals and managed directly by its 43 regional sales offices in China. Leveraging on its in-house R&D platform in growth factor and antibody, the Company maintains a pipeline of projects in various clinical stages, covering a wide range of fields and indications.






























































 粤公网安备 44049102496184号
粤公网安备 44049102496184号