Growth
Factor
Factor
Basic fibroblast growth factor ( bFGF ) is an active substance in trace amount existing in mammals and human body,and an important member of the FGF family-FGF2. Fibroblast growth factor (FGF) , an important member of the growth factor family is a cell signaling protein that plays an active role in the cell life cycle, including: cell proliferation, apoptosis, differentiation, survival, adhesion, exercise, Chemotaxis, neovascularization, homeostasis, etc.
Mechanism of action: Promote the growth and regulation of cells derived from mesoderm and neuroectoderm, and participate in tissue repair and wound healing;
Clinical application: Approved for the treatment of burn wounds, chronic ulcers, corneal injury repair, periodontitis and other indications. It is still in clinical research for fracture and nerve injury repair;
Drug characteristics: Active healing process improve healing efficacy and reduce complications;
bFGF Recombinant DNA Technology
Traditional biochemical extraction of bFGF is extremely difficult, as only one gram of bFGF can be extracted from per 10,000 cattle. The company has successfully modified and recombined the bovine bFGF and humanized bFGF based on the recombinant DNA technology, and obtained the highly effective expression plasmids of rb-bFGF and rh-bFGF and engineering bacteria for production, which fulfilled the requirments for mass production of bFGF.
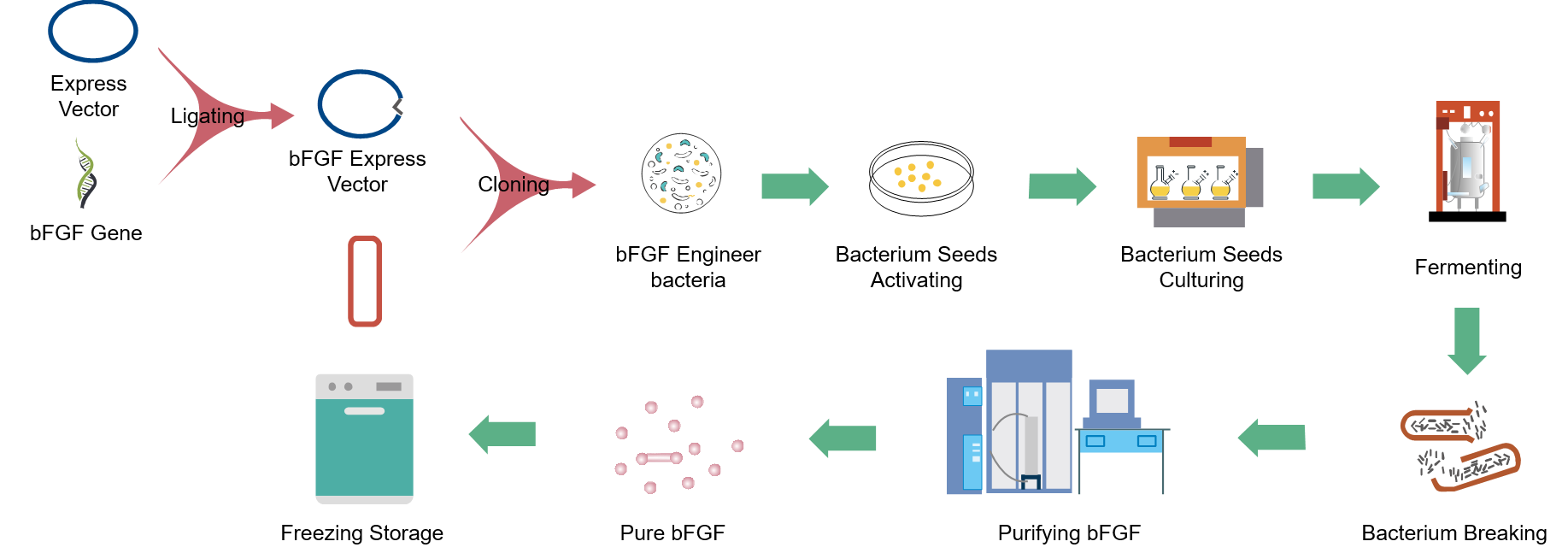
bFGF Large-scale Production Process and Quality Control Technology
The company developed and mastered the manufacturing technology for the high-density fermentation of rb-bFGF and rh-bFGF engineering bacteria, and the separation and purification of proteins, which satisfied the requirement for large-scale production of rb-bFGF and rh-bFGF. A comprehensive rb-bFGF and rh-bFGF API quality control system has been established.
bFGF Formulation Research and Production Technology
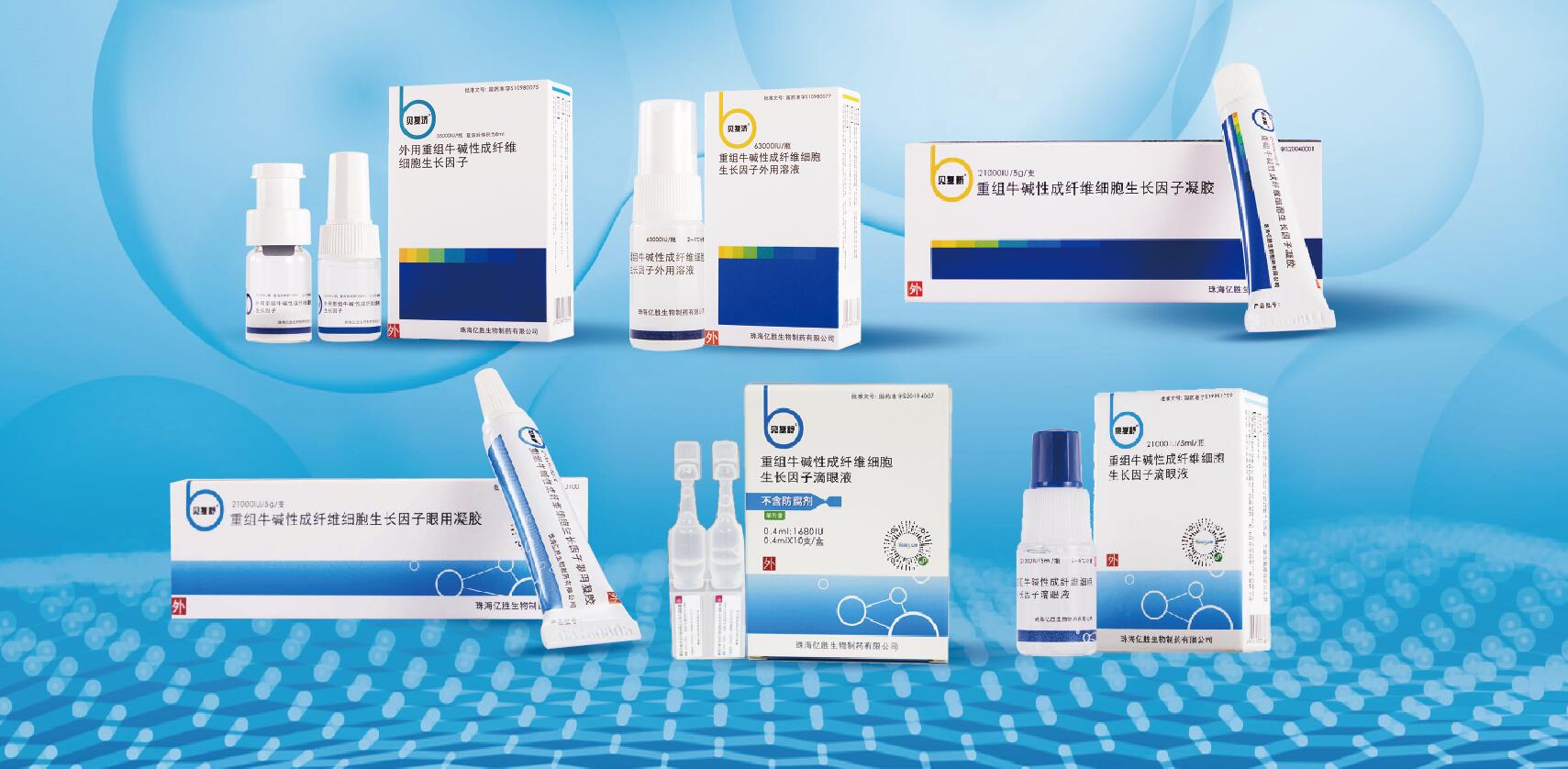
The company developed and mastered a series of core technologies including rb-bFGF and rh-bFGF formulations, production process and stability technology. Six rb-bFGF formulations have been successfully developed for treatment of ocular surface wounds and topical (skin) surface wounds respectively,out of which three are approved as Category I drugs.
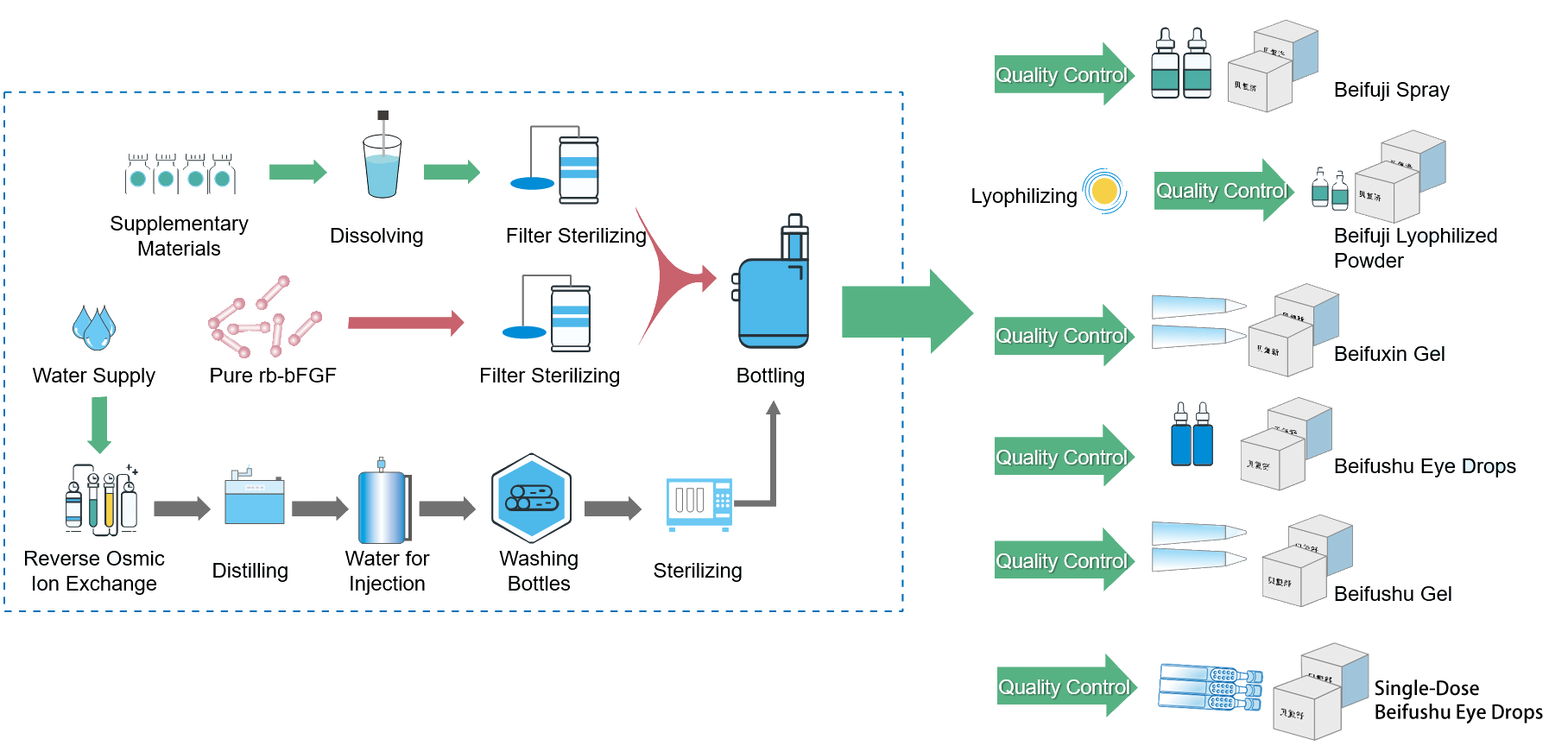
The product(s) from our FGF Technology Platform provides a therapeutics with balanced level of growth factor(s) to local damaged environment, making it a safe and effective treatment for human wounds and tissue disorders.
In recognition of Company's great contributions in research and development of quality control standards and standard products in the field of biopharmaceutical drugs, as well as its key technical breakthrough, theory innovation and industrialization for protein drugs of cellular growth factor in China, in 2004 and 2018, the Company was honoured with the second prize of "National Scientific and Technology Progress Award" issued by the State Council.

With continuous R&D efforts, the Company currently owns 34 patents, including 27 invention patents, 3 utility patents, 4 design patent, of which “rb-bFGF Eye Drops" was awarded one of the National Outstanding Innovation.

Globally, the clinical value of FGF is being affirmed by more and more authoritative experts. From 2009 to 2016, articles from renowned medical research journal focused on biology and applications of the FGFs family, underlined clinical value for therapeutics targeting FGF signaling through recombinant FGFs.
Guidelines recommended FGF for treatment of Ulcer Suppression Scars:
The American Wound Healing Association (WHS) and the European Wound Management Association (EWMA) recommended FGF for patients with Refractory Ulcers.
Japan's Burn and Plastic Surgery Guidelines describes the clinical application of bFGF in Scar Suppression.
For the future strategies of EssexBio in FGF-related drugs, we are working on evaluating optimization of recombinant humanized FGF-18 towards clinical indications and druggability,as well as assessing other potential therapeutic direction mediated by FGF/FGFR-tech platform (such as FGF-9, FGF-10, FGF-21)
Mechanism of action: Promote the growth and regulation of cells derived from mesoderm and neuroectoderm, and participate in tissue repair and wound healing;
Clinical application: Approved for the treatment of burn wounds, chronic ulcers, corneal injury repair, periodontitis and other indications. It is still in clinical research for fracture and nerve injury repair;
Drug characteristics: Active healing process improve healing efficacy and reduce complications;
bFGF Recombinant DNA Technology
Traditional biochemical extraction of bFGF is extremely difficult, as only one gram of bFGF can be extracted from per 10,000 cattle. The company has successfully modified and recombined the bovine bFGF and humanized bFGF based on the recombinant DNA technology, and obtained the highly effective expression plasmids of rb-bFGF and rh-bFGF and engineering bacteria for production, which fulfilled the requirments for mass production of bFGF.

bFGF Large-scale Production Process and Quality Control Technology
The company developed and mastered the manufacturing technology for the high-density fermentation of rb-bFGF and rh-bFGF engineering bacteria, and the separation and purification of proteins, which satisfied the requirement for large-scale production of rb-bFGF and rh-bFGF. A comprehensive rb-bFGF and rh-bFGF API quality control system has been established.
bFGF Formulation Research and Production Technology
The company developed and mastered a series of core technologies including rb-bFGF and rh-bFGF formulations, production process and stability technology. Six rb-bFGF formulations have been successfully developed for treatment of ocular surface wounds and topical (skin) surface wounds respectively,out of which three are approved as Category I drugs.

The product(s) from our FGF Technology Platform provides a therapeutics with balanced level of growth factor(s) to local damaged environment, making it a safe and effective treatment for human wounds and tissue disorders.

In recognition of Company's great contributions in research and development of quality control standards and standard products in the field of biopharmaceutical drugs, as well as its key technical breakthrough, theory innovation and industrialization for protein drugs of cellular growth factor in China, in 2004 and 2018, the Company was honoured with the second prize of "National Scientific and Technology Progress Award" issued by the State Council.

With continuous R&D efforts, the Company currently owns 34 patents, including 27 invention patents, 3 utility patents, 4 design patent, of which “rb-bFGF Eye Drops" was awarded one of the National Outstanding Innovation.

Globally, the clinical value of FGF is being affirmed by more and more authoritative experts. From 2009 to 2016, articles from renowned medical research journal focused on biology and applications of the FGFs family, underlined clinical value for therapeutics targeting FGF signaling through recombinant FGFs.
Guidelines recommended FGF for treatment of Ulcer Suppression Scars:
The American Wound Healing Association (WHS) and the European Wound Management Association (EWMA) recommended FGF for patients with Refractory Ulcers.
Japan's Burn and Plastic Surgery Guidelines describes the clinical application of bFGF in Scar Suppression.
For the future strategies of EssexBio in FGF-related drugs, we are working on evaluating optimization of recombinant humanized FGF-18 towards clinical indications and druggability,as well as assessing other potential therapeutic direction mediated by FGF/FGFR-tech platform (such as FGF-9, FGF-10, FGF-21)




 粤公网安备 44049102496184号
粤公网安备 44049102496184号